
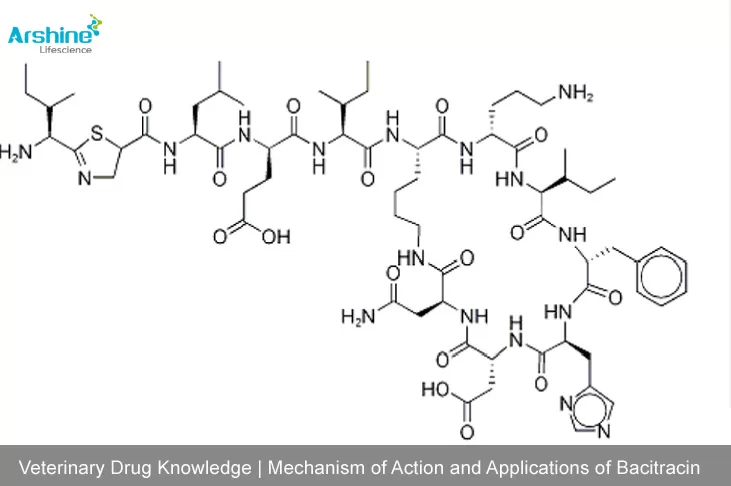
1. Historical Origin and Properties of Bacitracin
Bacitracin is a broad-spectrum polypeptide antibiotic containing a thiazole ring, first discovered in 1943 by Johnson at Columbia University from Bacillus subtilis isolated from a patient's wound.
Bacitracin exists in multiple isomers (A, B, C, D, E, F1, and G), with component A exhibiting the highest activity. Notably, component F is an oxidative metabolite with nephrotoxicity. The molecule contains both D- and L-amino acids, rendering it resistant to proteolytic enzymes (e.g., trypsin, pepsin), protease inhibitors, and bodily fluids.
However, bacitracin is inherently unstable and prone to degradation. Its stability and activity are enhanced when complexed with zinc ions, manganese ions, or methylenesalicylate. Thus, during production, zinc sulfate, zinc chloride, or methylenesalicylate is added to form more stable complexes: zinc bacitracin or methylenesalicylate bacitracin. These derivatives maintain potency even under acidic, alkaline, or high-temperature conditions when dried.
2. Pharmacological Actions
As a polypeptide antibiotic, bacitracin’s spectrum resembles penicillin, inhibiting:
Gram-positive bacteria (e.g., Clostridium, Staphylococcus),
Some Gram-negative bacteria (e.g., Neisseria meningitidis, Haemophilus influenzae),
Spirochetes and actinomycetes, with particular efficacy against Clostridium perfringens.
Triple Antibacterial Mechanism:
Inhibits cell wall synthesis: Blocks phospholipid carrier transport, disrupting peptidoglycan delivery.
Damages cell membranes: Increases permeability, causing leakage of ions, amino acids, and purines.
Disrupts intracellular protein synthesis, halting bacterial growth.
Additional Benefits:
Boosts intestinal immunoglobulin levels, enhancing immunity.
Activates TLR receptors and NF-κB signaling, promoting cytokine expression and innate immune responses.
3. Applications
Due to its instability, bacitracin is typically formulated as zinc bacitracin or methylenesalicylate bacitracin for practical use. These are primarily employed as:
Growth promoters in animal feed,
Therapeutics for Gram-positive bacterial infections (e.g., clostridial enteritis).
Low incompatibility: Combines synergistically with numerous antibiotics (e.g., penicillin G, streptomycin, neomycin, polymyxin) and 13 anticoccidials (e.g., amprolium, roxarsone, decoquinate).
Antagonistic interactions: Quinolones (e.g., olaquindox) and enramycin.
Bactericidal with low resistance risk: Rare cross-resistance development.
Metabolic fate: In the gut, it degrades into bacitracin (non-absorbed, localized action) and zinc ions (absorbed/utilized), minimizing residues in animal products.
Second-generation bacitracin: Larger molecular weight, superior intestinal solubility, and targeted small-intestinal activity without systemic absorption.
Expanded anticoccidial combinations: Compatible with diclazuril, halofuginone, and seduramicin.
Therapeutic uses:
Poultry: Controls Clostridium-induced necrotic enteritis.
Swine: Treats Brachyspira-associated dysentery; combined with chlortetracycline, it manages Lawsonia intracellularis (porcine proliferative enteropathy).
4. Conclusion
Bacitracin derivatives stand out in veterinary medicine due to their:
Rapid excretion,
Minimal residues/resistance,
Broad compatibility,
Economic benefits in livestock production.
Rational use (e.g., adhering to withdrawal periods or selecting non-absorbable variants like zinc bacitracin) ensures compliance with food safety regulations, fostering sustainable, healthy farming and high-quality animal products.
Add: Block 14, No.100, Luyun Road, Changsha 410205, Hunan, China.
Email: info@arshinevet.com
WeChat: +8618874001228
WhatsApp: +8615697311407
Tel:86-731-82294958